top of page

Search


Introducing Our New Webpage Interface
We are thrilled to announce the launch of our newly redesigned webpage interface! The updated design offers a clearer, more intuitive experience, allowing visitors to easily learn about our innovative technology while staying informed on the latest advancements and updates . This refresh reflects our ongoing commitment to accessibility, clarity, and connection; ensuring that every visitor can engage seamlessly with our content and explore the progress driving our work forwa

Biomedical Bonding
Nov 13, 20251 min read

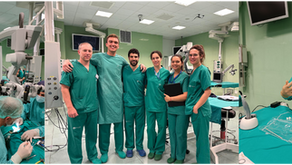
Pre-clinical Implantation study in collaboration with RISE, SÖS and CCMIJU
We are thrilled to announce the launch of our latest pre-clinical implantation study, conducted in collaboration with Research Institutes...

Biomedical Bonding
Jul 16, 20251 min read


Research paper on AdhFix published in Frontiers in Bioengineering and Biotechnology
The BoneFix team at the AO Research Institute Davos (ARI) has published another paper in Frontiers in Bioengineering and Biotechnology....

Biomedical Bonding
Jul 9, 20252 min read


Chapter 2 – Bonevolent™-AdhFix vs Metal Plates: A Comparative Look At Bone Fixation Technologies
The field of veterinary orthopaedics is constantly evolving, with new technologies emerging to improve fracture treatment. Traditionally,...

Biomedical Bonding
Mar 18, 20252 min read


Biomedical Bonding AB are happy to announce that Rami Elias has joined Biomedical Bonding AB as our new Chief Medical Officer and board member.
Member of the Board and Chief Medical Officer (CMO). Rami is a highly skilled Orthopedic Surgeon specializing in sports medicine as well...

Biomedical Bonding
Jul 26, 20241 min read


Biomedical Bonding AB Welcomes Mattias Ohrlander as COO
We are happy to announce that Mattias Ohrlander has joined Biomedical Bonding AB as our new Chief Operating Officer, effective May 15th,...

Biomedical Bonding
May 17, 20241 min read


Final validation assessment of Bonevolent™ - AdhFix
BMB delivers over 200 sterilized AdhFix products to Rise Research Institutes of Sweden for final biocompatibility and animal...

Biomedical Bonding
May 6, 20241 min read


BMB attends the AtiO conference 2024 in Berlin
BMB, represented by its Founder, Prof. Michael Malkoch, attended the ATiO 2024 meeting in Berlin to engage with top-tier surgeons and...

Biomedical Bonding
Apr 14, 20241 min read


Meet us in San Francisco at the AAOS Experience 2024!
Biomedical Bonding is one of the four finalists in Orthopitch 13th February - an event where innovative solutions are highlighted. We are...

Biomedical Bonding
Jan 25, 20241 min read


Finalist AAOS 2024
The American Academy of Orthopedic Surgeons (AAOS) has selected Biomedical Bondings as one of the four finalists for the upcoming...

Biomedical Bonding
Nov 8, 20231 min read


New publication – AdhFix mechanically superior
Do not miss this intriguing publication in this fine magazine, Veterinary Surgery 2023:1-9! Here, AdhFix was tested and found to be...

Biomedical Bonding
Nov 7, 20231 min read


New article on surgeons constructing the customisable composite fixation plate Adhfix
The BoneFix project builds on light-cured on-site customisable implant innovations that have the potential to improve the surgical...

Biomedical Bonding
Oct 5, 20231 min read


Granted patent US
Biomedical Bonding has received a granted patent from the United States Patent and Trademark Office (USPTO). The invention relates to a...

Biomedical Bonding
Aug 10, 20231 min read


A new scientific report published from the Bonefix project!
According to this study, AdhFix is considered a promising novel technology that provides a readily customizable solution for fracture...

Biomedical Bonding
Jun 16, 20231 min read



Biomedical Bonding
Jun 16, 20230 min read


Meet us at the Medtech Forum in Dublin!
Biomedical Bonding joins Medtech Forum in Dublin. Let’s connect, discuss, and exchange ideas and future opportunities in the medical...

Biomedical Bonding
May 26, 20231 min read


Cleanroom manufacturing of Bonevolent™ AdhFix
During the spring, Chief Technology Officer Viktor Granskog along with product engineers Lola Opande and Ram Shadher, have been stationed...

Biomedical Bonding
May 17, 20231 min read


Meet us at LSX, 3-4 May 2023 in London.
Biomedical Bonding will participate in LSX World Congress and present a paradigm shift in bone restoration surgeries – a biocompatible...

Biomedical Bonding
Apr 26, 20231 min read


Notice of Allowance - patent protection US
Notice of Allowance - patent protection US

Biomedical Bonding
Mar 1, 20231 min read


EPO announces intention to grant BMB an additional patent
EPO announces intention to grant BMB an additional patent

Biomedical Bonding
Feb 24, 20231 min read
Latest NEWS
bottom of page
